 A paper from the lab of Pei Tang, PhD, “Structures of Highly Flexible Intracellular Domain of Human α7 Nicotinic Acetylcholine Receptor,” was published in the high-impact journal Nature Communications (doi: 10.1038/s41467-022-28400-x). The study was conducted in partnership with Yan Xu, PhD, and collaborators at Stockholm University, the KTH Royal Institute of Technology in Solna, Sweden, and the Department of Chemistry at the University of Pittsburgh.
A paper from the lab of Pei Tang, PhD, “Structures of Highly Flexible Intracellular Domain of Human α7 Nicotinic Acetylcholine Receptor,” was published in the high-impact journal Nature Communications (doi: 10.1038/s41467-022-28400-x). The study was conducted in partnership with Yan Xu, PhD, and collaborators at Stockholm University, the KTH Royal Institute of Technology in Solna, Sweden, and the Department of Chemistry at the University of Pittsburgh.
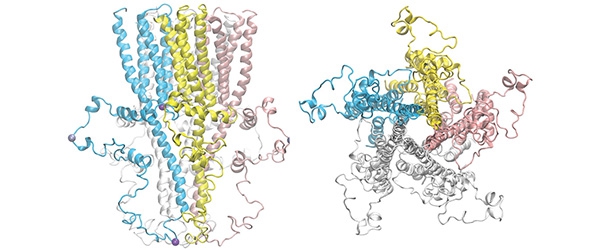
The intracellular domain (ICD) of the so-called Cys-loop receptors mediates diverse functions, but no complete structure of a Cys-loop receptor ICD was previously available due to challenges stemming from its dynamic nature. In their paper, the authors determined the full-length ICD structures of the human α7 nicotinic acetylcholine receptor (α7nAChR), a major subtype of neuronal nAChRs expressed in the brain and immune cells. Dr. Tang et al. used an innovative approach by combining nuclear magnetic resonance and electron spin resonance spectroscopy with Rosetta computations to solve this structure.
The success of this work by Dr. Tang’s team breaks new ground. The techniques used in their study can be applied to solve the ICD structures of other neurotransmitter-regulated receptors and other structures that can’t be determined using X-ray crystallography and cryogenic electron microscopy. Furthermore, solving the first high-resolution structure of the α7nAChR ICD finally opens new doors to search for next-generation drugs to treat diseases and pathological conditions such as addiction, Alzheimer’s, autism, cytokine storm, and long COVID.
The article was featured on the Nature Communications Structural Biology, Biochemistry, and Biophysics Editors’ Highlights webpage. Editor’s Highlights showcases the 50 best papers recently published in an area and routinely replaces papers as more work is published in the journal.
